The influence of pores in activated carbon on ultra dense hydrogen storage
Molecular hydrogen (H2), as a potential zero carbon energy carrier for sustainable development, is abundant in the form of water and biomass, relatively easy to produce, such as through water electrolysis or thermochemical treatment of biomass, and has little pollution. Hydrogen has a relatively high calorific value, but it exists as an extremely low-density gas in the environment, resulting in a lower volumetric energy density compared to liquid fuels. Therefore, gaseous hydrogen must be dense for effective storage and transfer. The general method is to pressurize or liquefy hydrogen gas to increase its volumetric energy density. Another method is to use activated carbon to adsorb and store hydrogen gas.
In hydrogen storage materials, molecular confinement involves van der Waals interactions between adsorbed hydrogen and pore surfaces, and is amplified for small pores due to overlapping potentials from relative pore walls. Under supercritical conditions, different pore geometries of activated carbon may affect hydrogen adsorption and densification. So this experiment studied three types of activated carbon with different pore geometries to analyze how the difference in hydrogen molecule accumulation between slit shaped and cylindrical pores affects the efficiency of hydrogen densification.
Pore analysis of activated carbon
Three types of activated carbon were selected, representing three different pore geometries while retaining chemically uniform adsorption surfaces and comparable pore sizes. The selected samples are activated carbon composed of randomly arranged graphite layers, titanium carbide derived activated carbon with slit pore geometry, and activated carbon nanotube samples with cylindrical pore geometry. Heat treat activated carbon nanotubes to remove end caps and allow gas to enter the internal pores. Select heating conditions to adjust the porosity and BET surface area of activated carbon nanotubes to a level comparable to the other two carbon samples, while also cleaning the surface and removing impurities. The characteristics of all three samples are as follows: the differences in pore geometry of these materials were demonstrated using transmission electron microscopy (TEM) Figure 1.
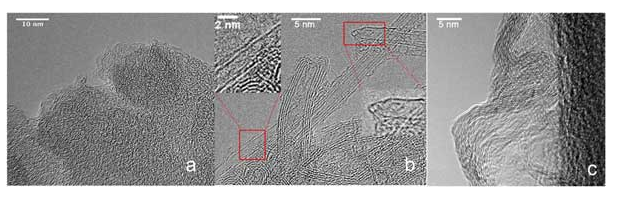
Figure 1: (a) Graphite activated carbon, (b) Activated carbon nanotubes, (c) Titanium activated carbon (slit shaped pores).
Testing method for hydrogen storage pores of activated carbon
The activated carbon sample is degassed by non in situ heating under high vacuum for more than 10 hours, and then placed in a glove box and a high-pressure stainless steel sample tank. The temperature is controlled by standard low-temperature furnace auxiliary equipment. Prior to hydrogen injection, background scan images of degassed samples under dynamic vacuum were collected (each scan lasting approximately 4 hours). Then subtract the background scan from the INS spectrum to correct for the presence of terminal H atoms in the sample. We used inelastic neutron scattering and in-situ gas quantitative injection to experimentally investigate the effect of confinement conditions on the behavior of very light hydrogen molecules in activated carbon pores under pressure at low temperatures. Neutrons have high permeability and can be used in high-pressure and low-temperature sample environments. In the case of hydrogen physical adsorption, they can be used to observe the rotational transitions of adsorbed molecular hydrogen, providing information about the state of hydrogen in activated carbon pores (gas, liquid, or solid).
Non elastic neutron scattering and hydrogen adsorption
In the inelastic neutron scattering experiment, the in-situ hydrogen dose of ordinary hydrogen on three carbon atoms was used (Figure 2). Figure 2a shows the INS spectrum of hydrogen gas in titanium activated carbon at a pressure of 0.3 MPa, indicating a clear peak at 0 meV attributed to the elastic and quasi elastic scattering of fixed (solid) and partially mobile (liquid) hydrogen. The spectrum a viewed on the logarithmic energy axis in Figure 2 shows the rotational peak of H2 adsorbed on activated carbon at 14.7 meV under different pressure loads. We previously observed a rotor peak on graphite activated carbon, which corresponds to hydrogen transitioning from opposite or forward rotation in the solid. The presence of a 14.7meV peak indicates that hydrogen molecules are in the following state: they are fixed in three dimensions and exhibit a free rotor, thus approximating a solid.
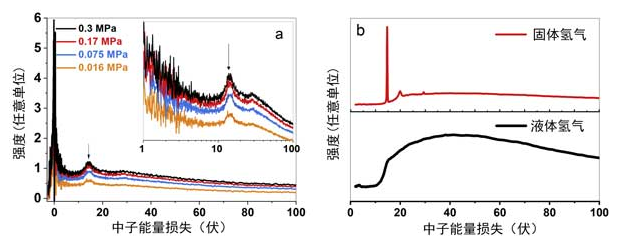
Figure 2: (a) INS spectrum of hydrogen on titanium activated carbon. (b) The INS spectra of thermal neutrons collected from the neutron database on liquid versus H2 (black line) and solid versus H2 (red line).
Molecular Dynamics Simulation
In order to elucidate the differences in the density of adsorbates, molecular dynamics simulations were conducted to explore the adsorption of hydrogen molecules within the micropores of activated carbon. The pore size and geometric shape were consistent with the experimental results. We simulated the density of H2 and the degree of closure at saturation, and found that for the 0.7nm pore, the geometric shapes of both types of pores are equivalent. Predict hydrogen density as solid. Molecular dynamics simulations have shown that hydrogen molecules form two highly ordered and well-defined layers within narrow slit shaped pores with a pore size less than 1nm (Figure 3). We observed a phase transition of H2 in the gap shaped pores, with a width of 0.5-0.65 nm. Through experiments, we demonstrated the formation of a solid with a bilayer structure and ordered ortho-H2 coordination. Hydrogen adsorption with activated carbon.
Figure 3: The simulation results show good hydrogen accumulation and orientation, with hydrogen confined to narrow slit shaped pores with a pore size of 0.66nm and pores with pore sizes of 0.60nm, 0.99nm, and 1.16nm. Hydrogen molecules are represented by a single gold ball surface gray line representing activated carbon atoms.
The influence of pores in activated carbon on ultra dense hydrogen storage has been confirmed through research. Even in the presence of weakly interacting molecules such as hydrogen, confinement in the pores can have a strong impact on gas/liquid and liquid/solid phase transformations. By combining in-situ INS, high-pressure gas adsorption experiments, and simulations, we systematically studied the effects of pore geometry and pore size on the density and migration rate of H2 in activated carbon, and compared and contrasted narrow slits. The pores in titanium activated carbon, the cylindrical pores in activated carbon nanotubes, and the disordered structure of activated carbon. For all pore geometries, it was found that hydrogen behavior is strongly influenced by small pore sizes (<1nm). Therefore, the narrow pore size distribution (<1nm) remains the most critical factor for increasing hydrogen density and the capacity of porous activated carbon materials. It is particularly recommended that during industrial manufacturing, adjusting the pore size of activated carbon to less than 1nm should be the main consideration for gas adsorption, rather than expensive control of uniform pore shape. In summary, this study provides new insights into the restricted hydrogen behavior under supercritical conditions. The results indicate that pore size remains a key factor in optimizing hydrogen density in activated carbon pores for high-capacity hydrogen storage applications. However, the geometry of the pores may represent another consideration for high-density gas-phase transitions in other applications, such as controlling hydrogen crystallization.

 CN
CN